Stephen V Liu, MD on Twitter: "The #ESMOVirtualPlenary presented by Dr. @LuisPaz_Ares presents results from EORTC-1416-LCG / ETOP 8-15 / PEARLS / KEYNOTE-091: adjuvant pembrolizumab versus placebo for resected early stage #NSCLC. @

Aakash Desai, MD, MPH on Twitter: "@LuisPaz_Ares discussed data from #PEARLS study #Pembrolizumab for early stage #NSCLC #LCSM with dual primary endpoint of DFS in all comers/PDL1>50%, showing significant DFS improvement HR:0.76
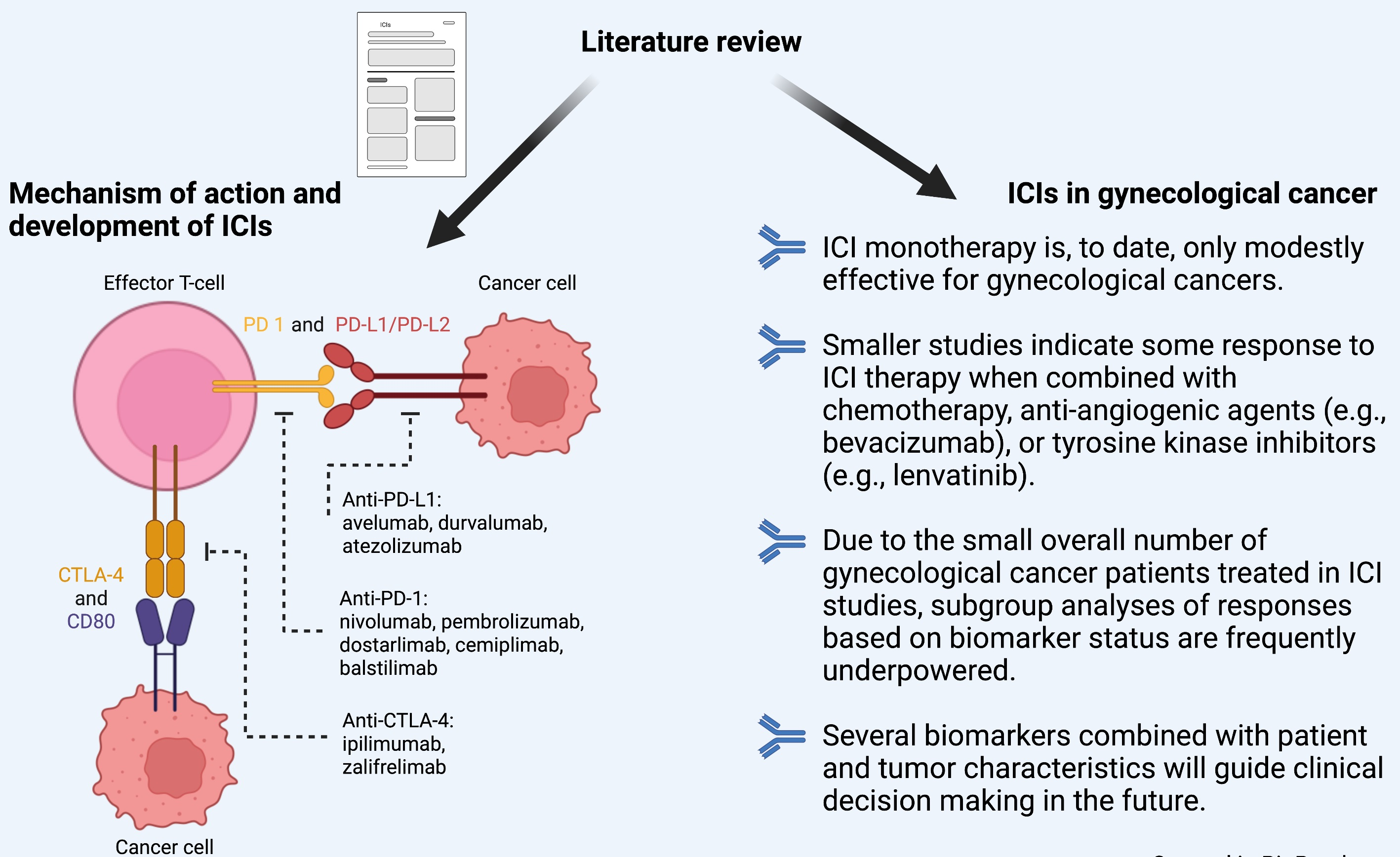
Cancers | Free Full-Text | Overview of Immune Checkpoint Inhibitors in Gynecological Cancer Treatment

Stephen V Liu, MD on Twitter: "#ASCO22 Discussion of PEARLS (KEYNOTE-091, @ETOP_eu 8-15), a phase III study of adjuvant pembrolizumab in resected NSCLC. The study did meet its primary endpoint (DFS HR
KEYTRUDA® (pembrolizumab) Showed Statistically Significant Improvement In Disease-Free Survival Versus Placebo As Adjuvant Treatment For Patients With Stage IB-IIIA Non-Small Cell Lung Cancer Regardless Of PD-L1 Expression 2023 - EORTC

KEYTRUDA® (pembrolizumab) Showed Statistically Significant Improvement In Disease-Free Survival Versus Placebo As Adjuvant Treatment For Patients With Stage IB-IIIA Non-Small Cell Lung Cancer Regardless Of PD-L1 Expression 2023 - EORTC
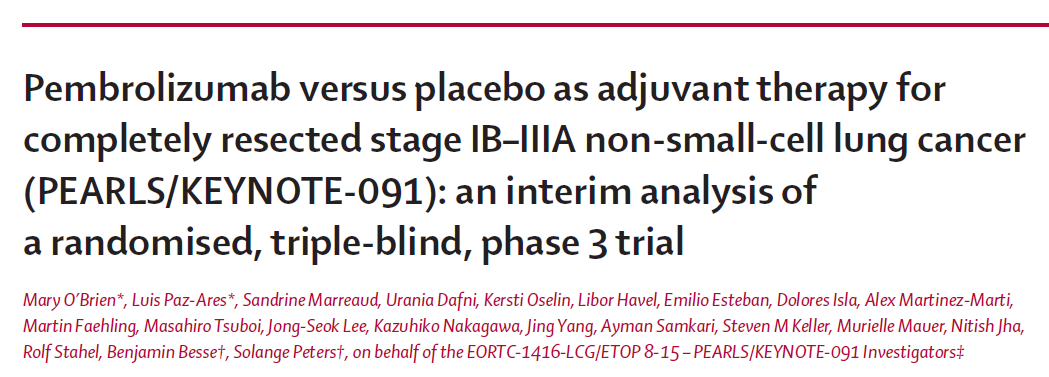
Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB–IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial | AIOM

Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB–IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial | AIOM
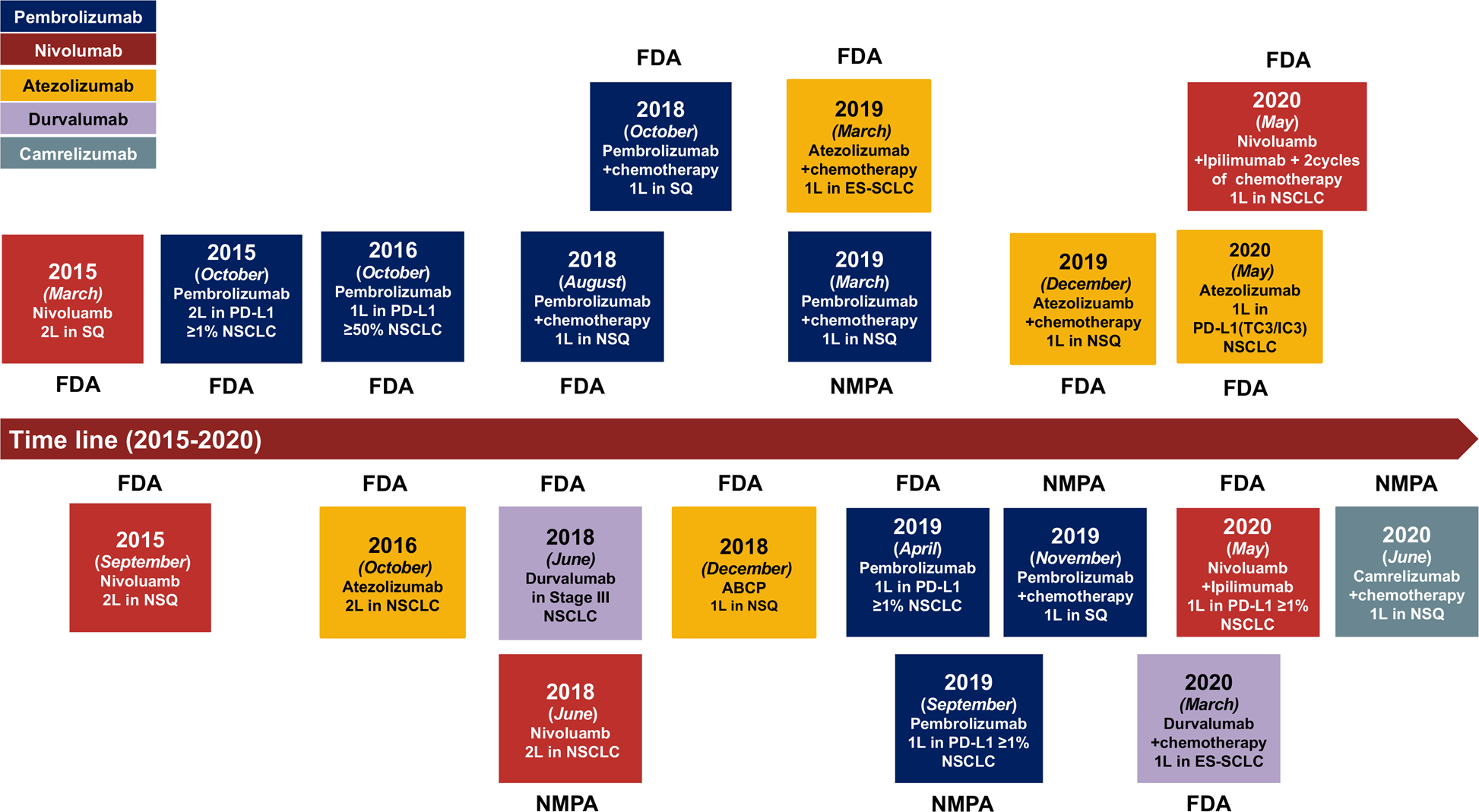
The cutting-edge progress of immune-checkpoint blockade in lung cancer | Cellular & Molecular Immunology

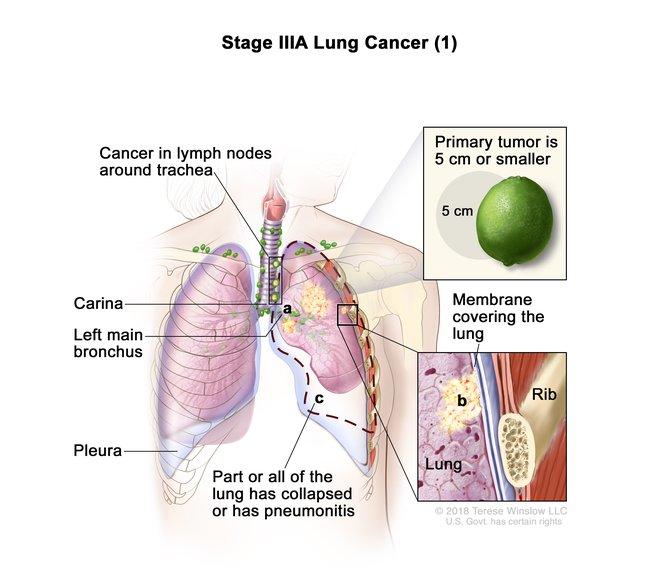
Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB–IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial - The Lancet Oncology

Benjamin Besse on Twitter: "Adjuvant pembrolizumab improves Disease Free Survival in pts after complete resection of stage IB-IIIA Non-Small Cell Lung Cancer HR=0.76 (IC95 0.63-0.91), including subgroups PD-L1 neg (HR=0.78) or stage

Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB–IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial - The Lancet Oncology
Frühes nichtkleinzelliges Bronchialkarzinom: Pembrolizumab adjuvant nach Resektion verlängert das krankheitsfreie Überleben