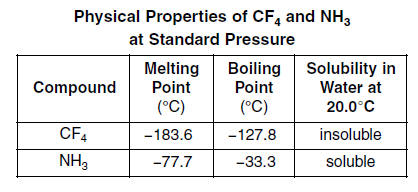
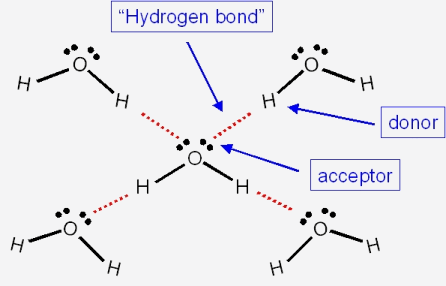
Among the following, which has the highest boiling point? (A) NH3 (B) PH3 (C) AsH3 (D) CH - Brainly.in

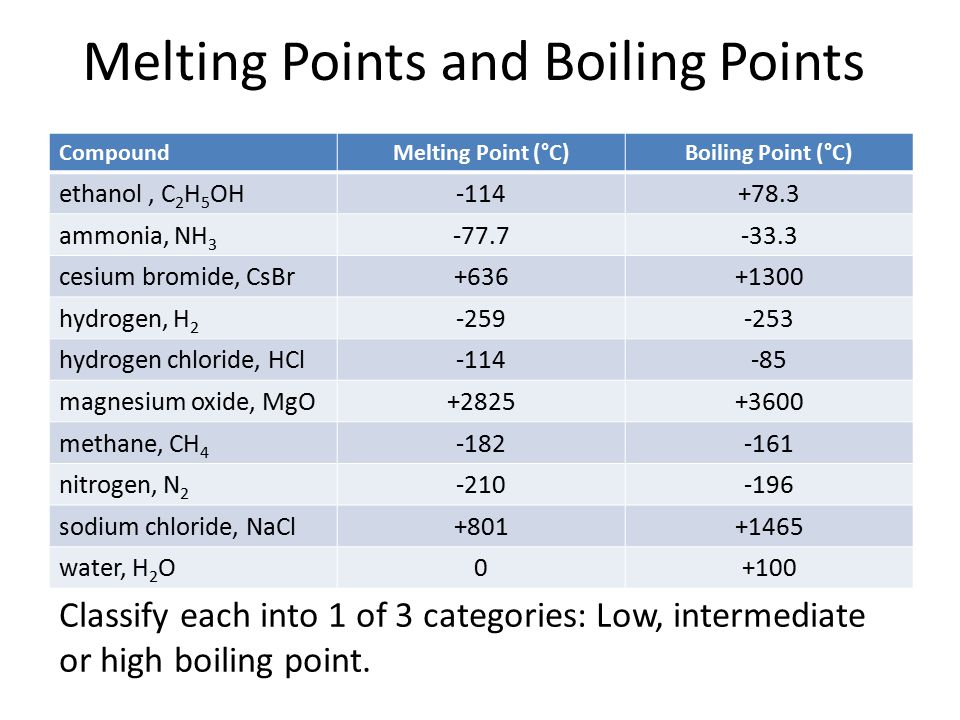
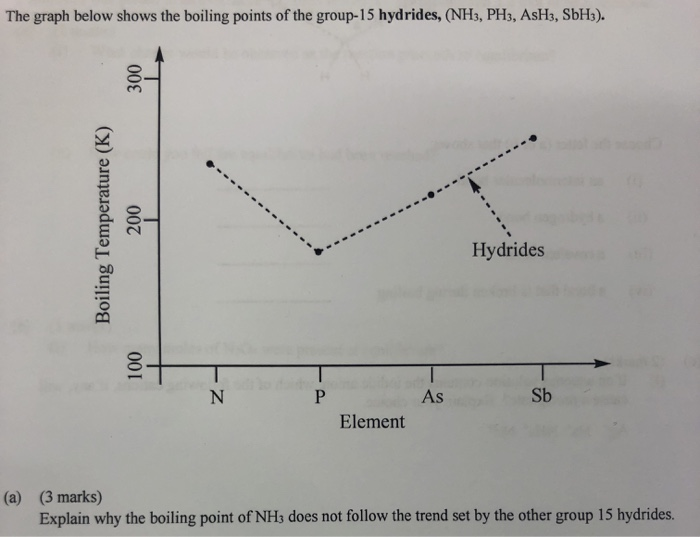
The hydrides of group 5A are NH3, PH3, AsH3, and SbH3. Arrange them from highest to lowest boiling point. - Brainly.com
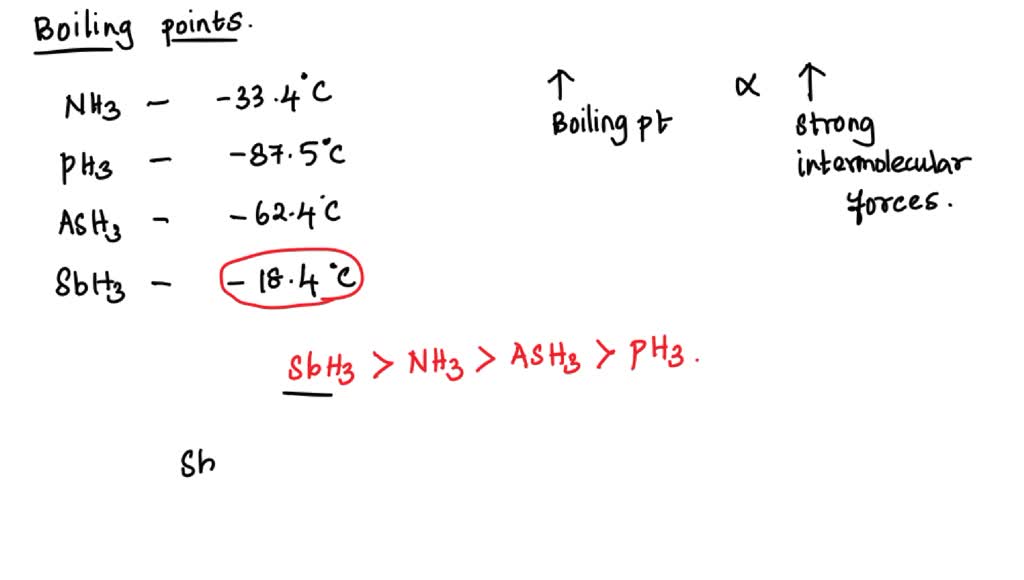
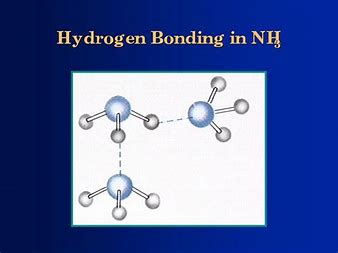
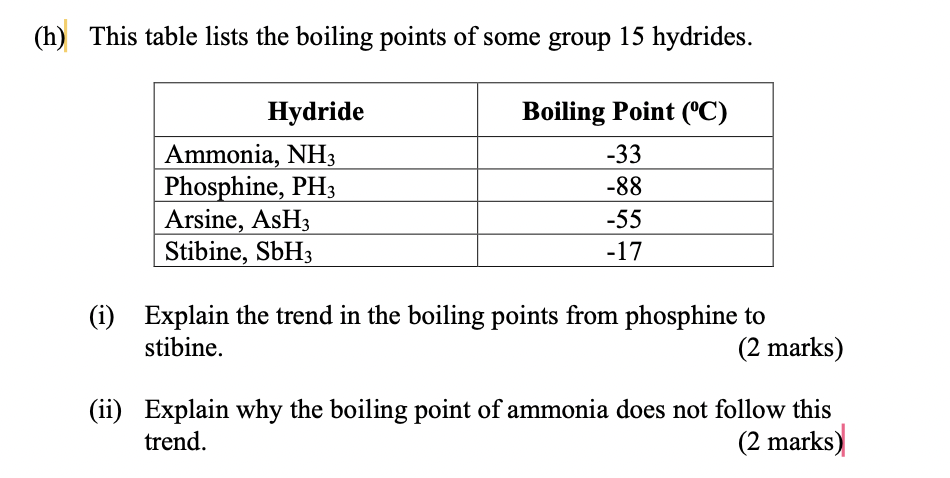
SOLVED: The boiling point of NH3, PH3,AsH3 and SbH3 are respectively -33.4 oC,-87.5 oC, -62.4 oC, -18.4oC. Explain the variation of their boiling points in terms of the types of intermolecular forces.