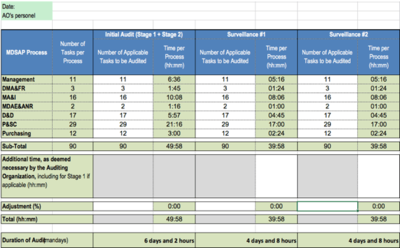
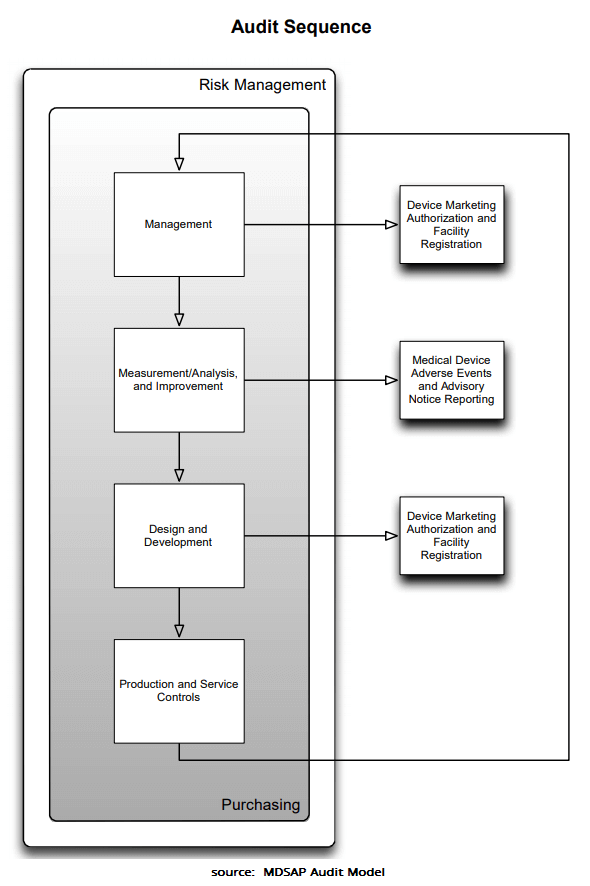
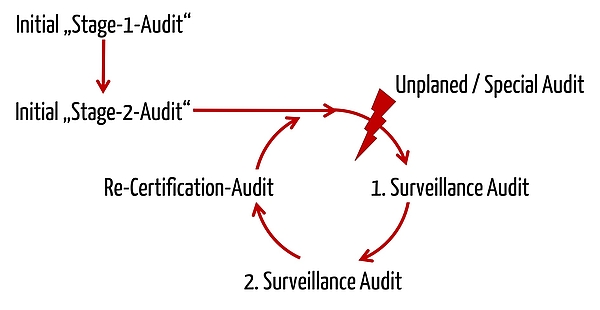
Freyr Medical Devices Regulatory Services on LinkedIn: Medical Device Single Audit Program (MDSAP) - Audit Processes

Medical Device Single Audit Program (MDSAP) – Chapters 1 to 4 - LearnGxP: Accredited Online Life Science Training Courses

The 7 Process Chapters of the MDSAP Audit Roadmap - LearnGxP: Accredited Online Life Science Training Courses