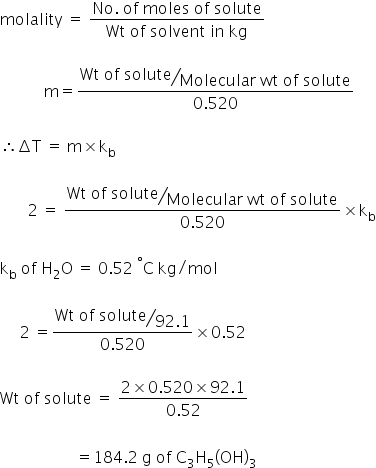EFFECTS OF ADDITION GLYCEROL CO-PRODUCT OF BIODIESEL IN THE THERMOPHYSICAL PROPERTIES OF WATER-GLYCEROL SOLUTION APPLIED AS SECO

Boiling points of the propylene glycol + glycerol system at 1 atmosphere pressure: 188.6-292 °C without and with added water or nicotine. - Abstract - Europe PMC
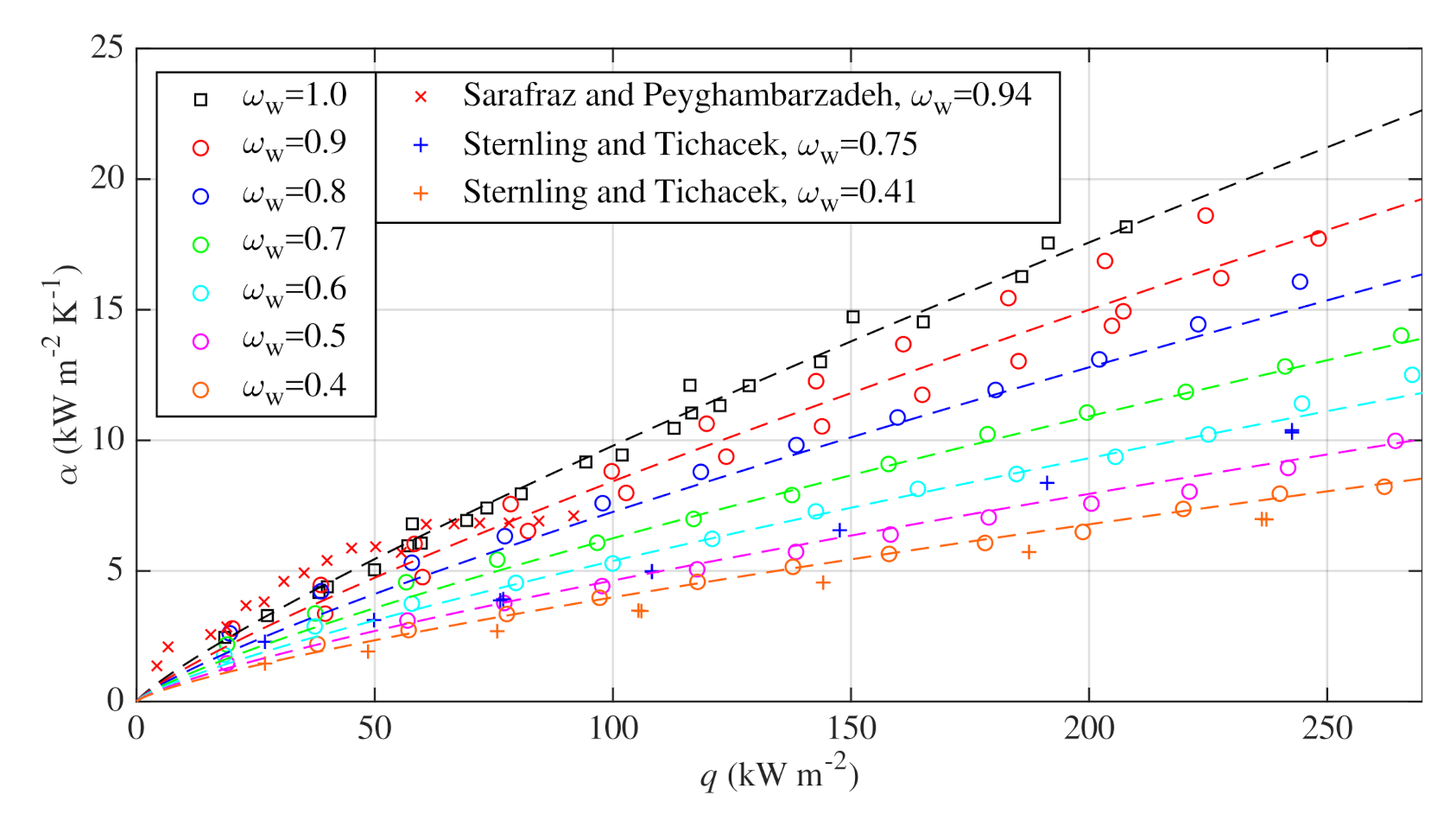
Processes | Free Full-Text | Pool Boiling Heat Transfer Coefficients in Mixtures of Water and Glycerin
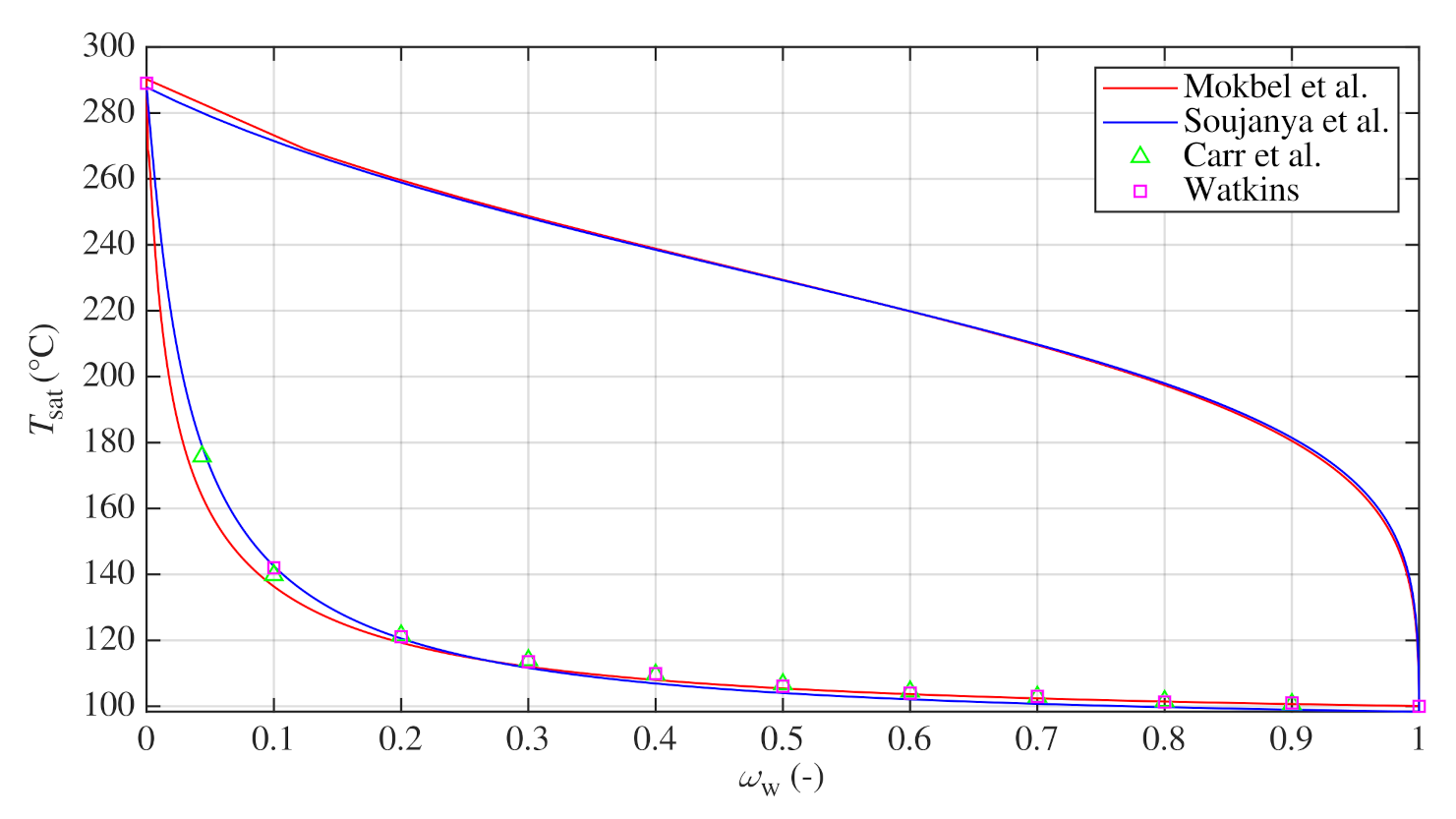
Processes | Free Full-Text | Pool Boiling Heat Transfer Coefficients in Mixtures of Water and Glycerin

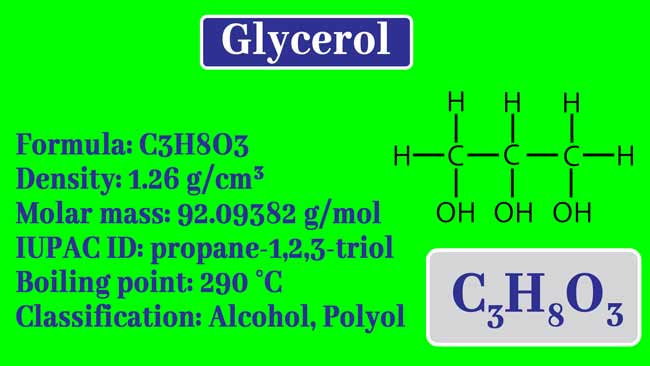
A solution of glycerol `(C-(3)H_(8)O_(3)` , molar mass = 92 g `mol^(-1)` iin water was prepared by - YouTube
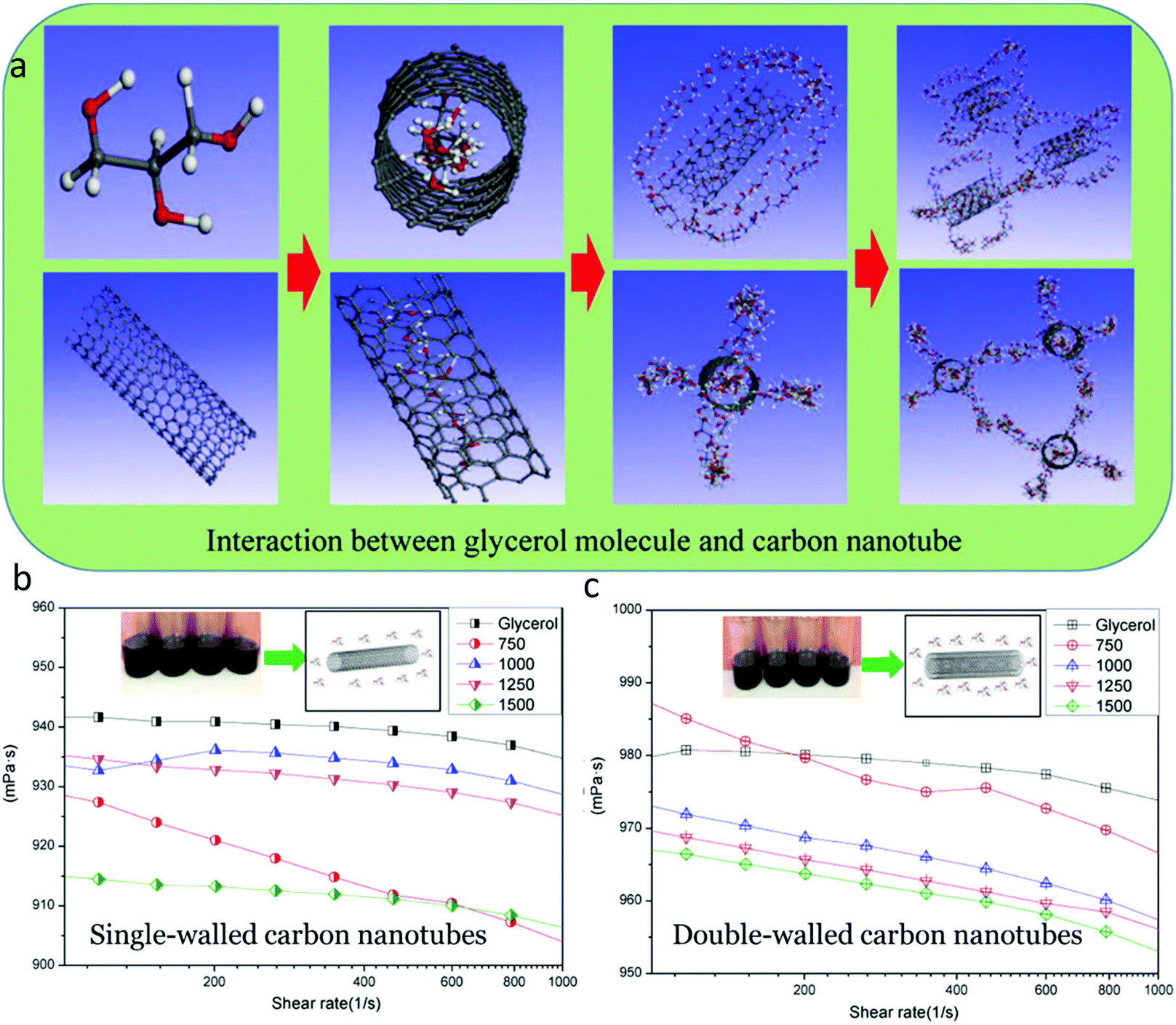
Glycerol in energy transportation: a state-of-the-art review - Green Chemistry (RSC Publishing) DOI:10.1039/D1GC02597J
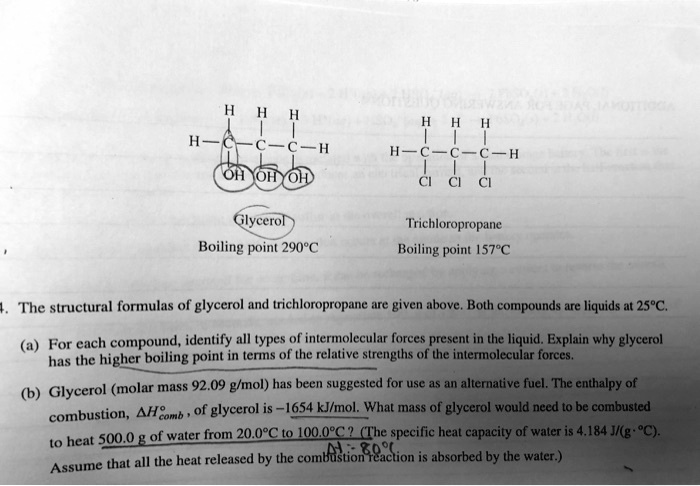
SOLVED: Glycerol Trichloropropane Boiling point 1578€ Boiling point 290"€ The structural formulas of glycerol and trichloropropane are given above. Both compounds are liquids at 258C. () For each compound, identify all types

Phase diagram of glycerol-water mixture with temperature (T/ • C) vs.... | Download Scientific Diagram

Glycerol decomposes at its boiling point, the purification of glycerol can be affected by | 12 ... - YouTube

SOLVED: Using the data provided below, calculate the boiling point (in oC) of a 0.22 molal solution of glycerol (C3H8O3) in ethanol (C2H6O). ethanol normal boiling point = 78.4 oC; Kb (oC/molal) =