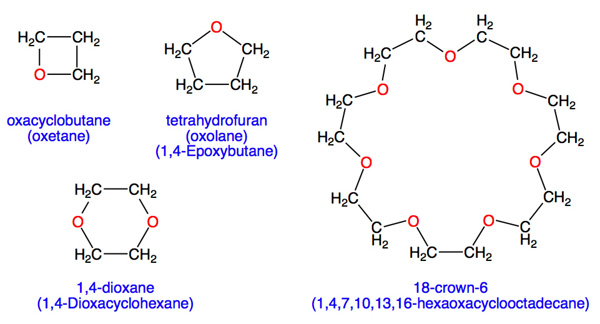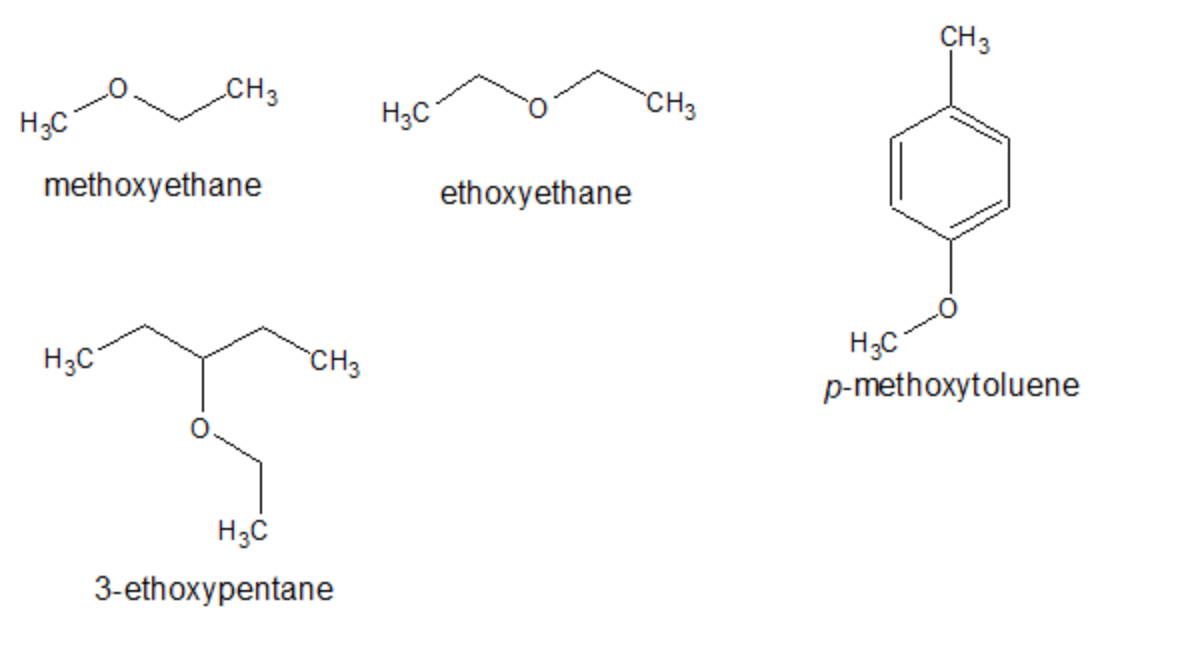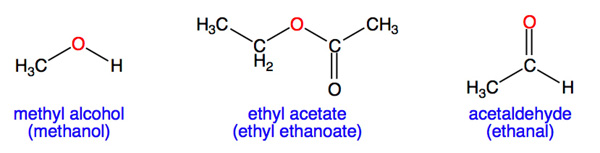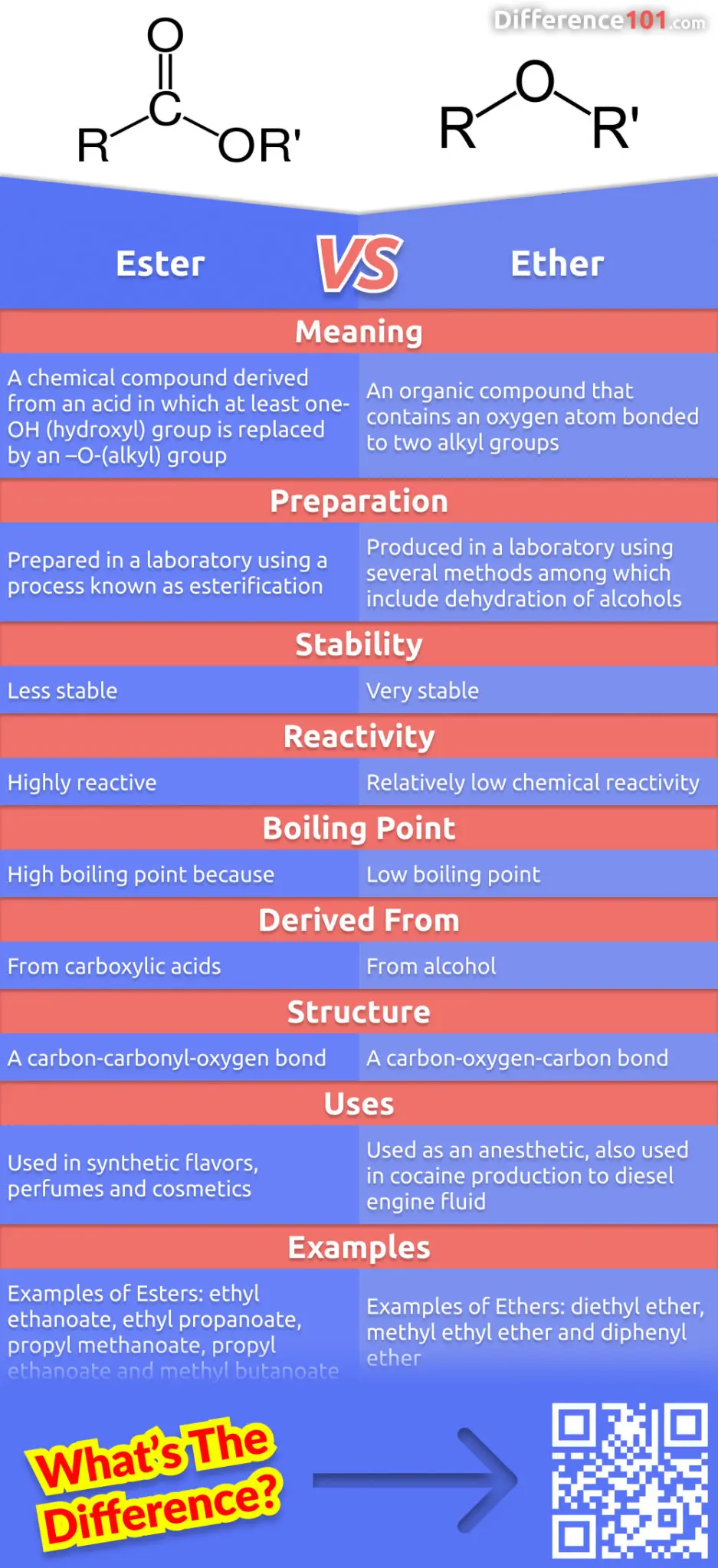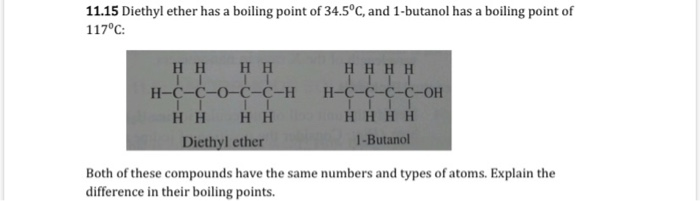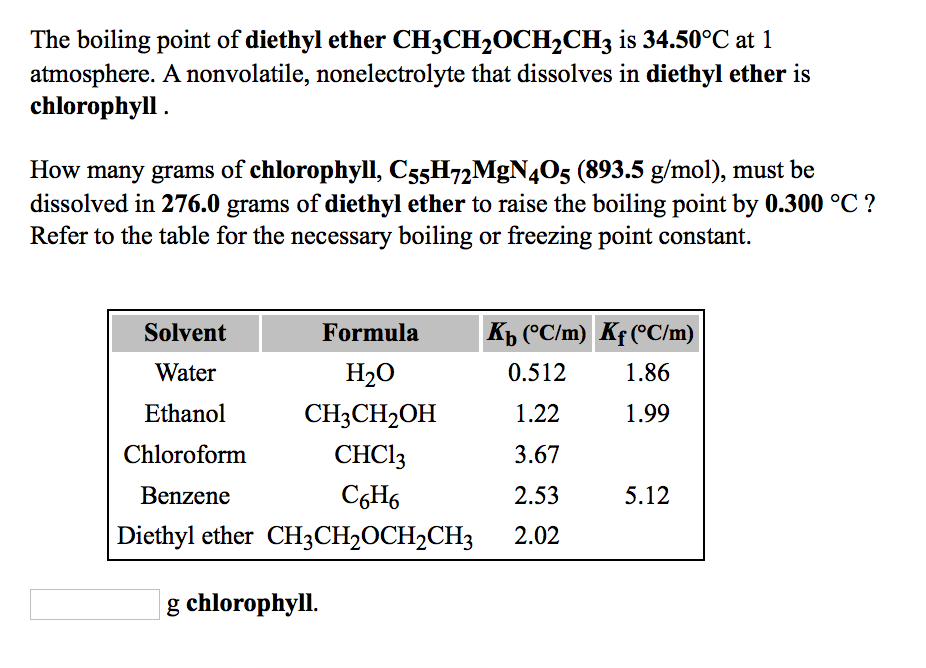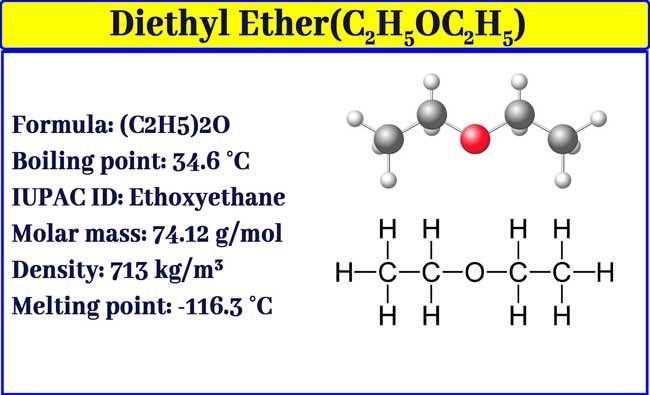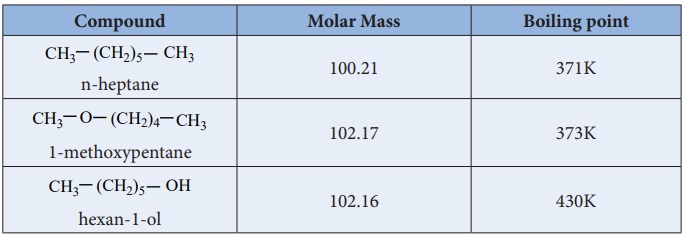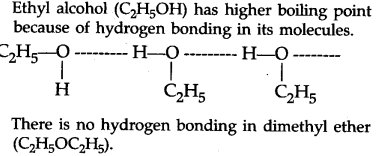
Which one has high boiling point ethyl alcohol or dimethyl ether and why? - CBSE Class 11 Chemistry - Learn CBSE Forum

Diethyl ether has a much higher boiling point than butane despite having a higher molecular weight. Explain why this is the case, making reference to the molecular structures of both compounds.

In general, ethers have a much lower boiling point than their isomeric alcohols. Why? A. The carbon-oxygen bond in ethers is nonpolar. B. Unlike alcohols, ethers cannot act as Lewis bases. C.
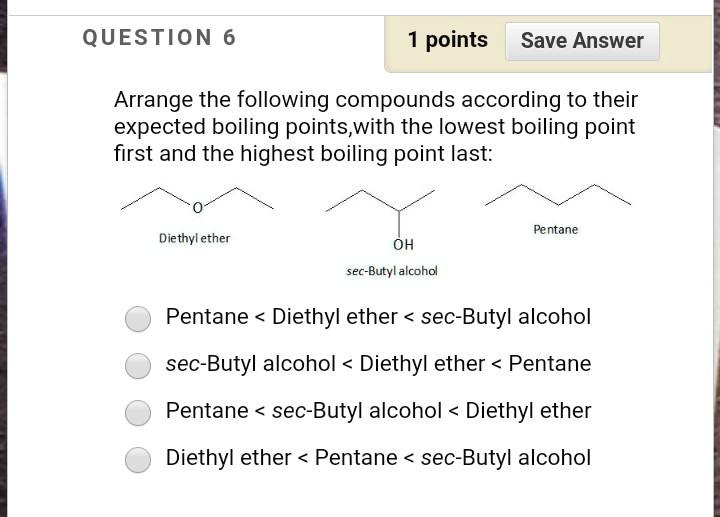
SOLVED: QUESTION points Save Answer Arrange the following compounds according to their expected boiling points,with the lowest boiling point first and the highest boiling point last: Pentane Diethyl ether OH sec-Butyl Icohol

Properties of Alcohols, Ethers, and Thiols Chapter 12 Organic Compounds with Oxygen and Sulfur Copyright © 2005 by Pearson Education, Inc. Publishing. - ppt download


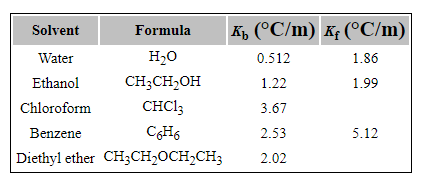

![Q34P N-Methylpyrrolidine has a boilin... [FREE SOLUTION] | StudySmarter Q34P N-Methylpyrrolidine has a boilin... [FREE SOLUTION] | StudySmarter](https://studysmarter-mediafiles.s3.amazonaws.com/media/textbook-exercise-images/image_EpmNdXt.png?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIA4OLDUDE42UZHAIET%2F20230602%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20230602T051144Z&X-Amz-Expires=90000&X-Amz-SignedHeaders=host&X-Amz-Signature=151f908f62dccfc03b59a3a5657a4b23afa52e38541bae40451932890437bd1c)