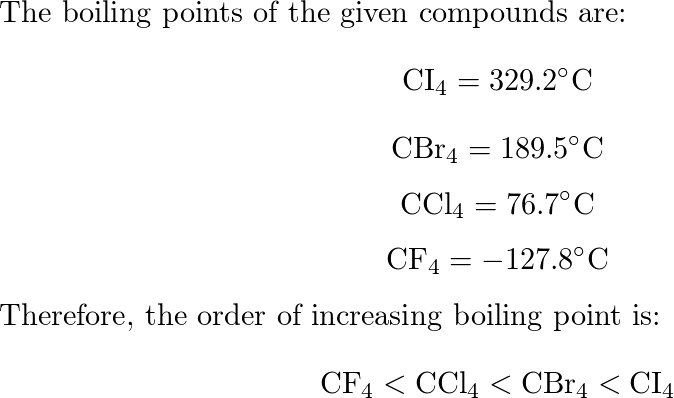SOLVED: Place the substances in order of decreasing boiling point: BeClz CBr4 BeBrz Select one: BeClz CBra BeBr2 b. BeClz > BeBr2 CBr4 CBra BeBrz BeClz d. BeClz BeBrz CBra BeBr2 BeClz

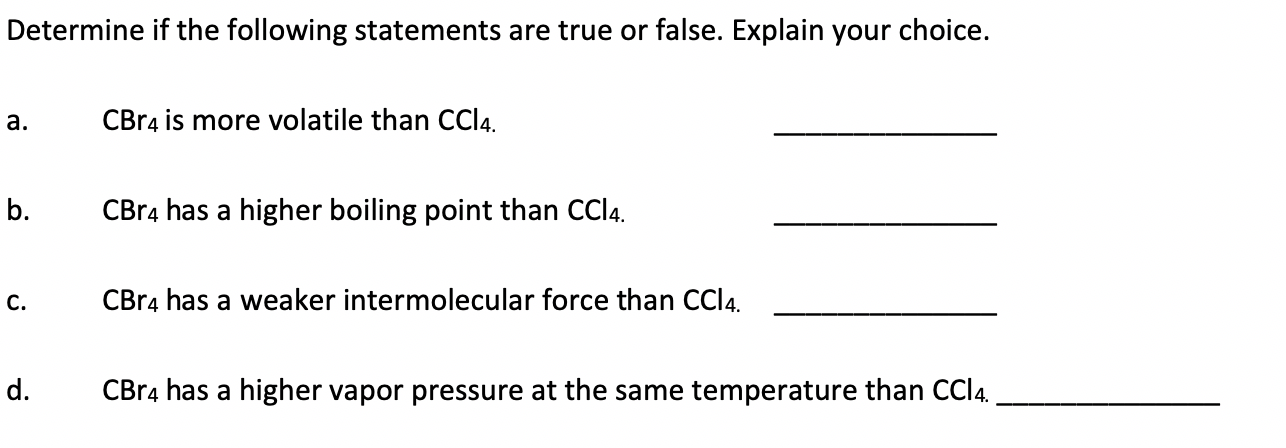
SOLVED: C) The boiling point of material is an indicator of the strength of the bonds of the compound: In this case, both CCl4 and CBr4 are covalent molecules with single bonds

SOLVED: CBr4 has a zero dipole moment and a boiling point of 189.5⁰C; CH3Br has a dipole moment of 0.05 D and a boiling point of 3.56⁰C. Briefly explain why this polar

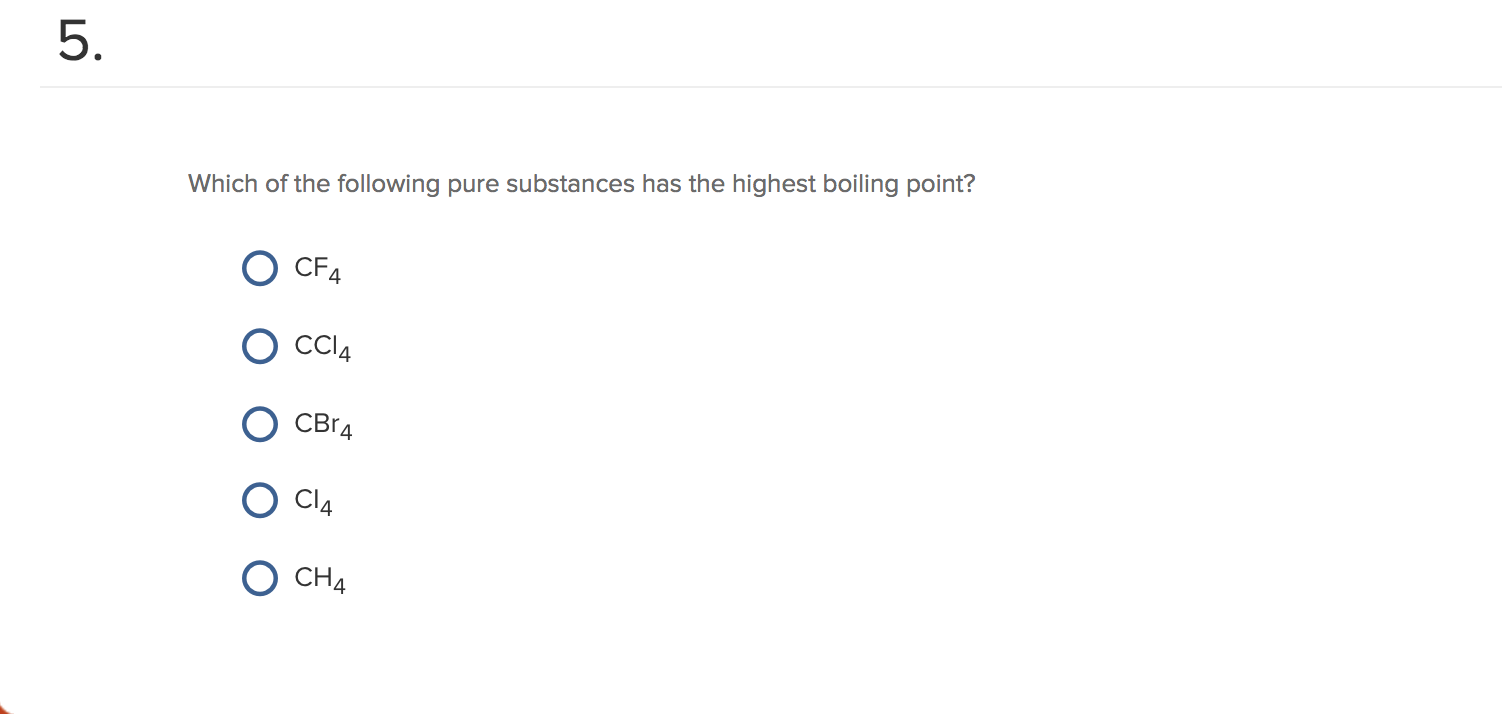
SOLVED: Rank the following compounds from lowest to highest boiling point: A. CI4 CBr4 C.CCl4 CF4 (Enter the letter corresponding to each compound ) Boiling point:
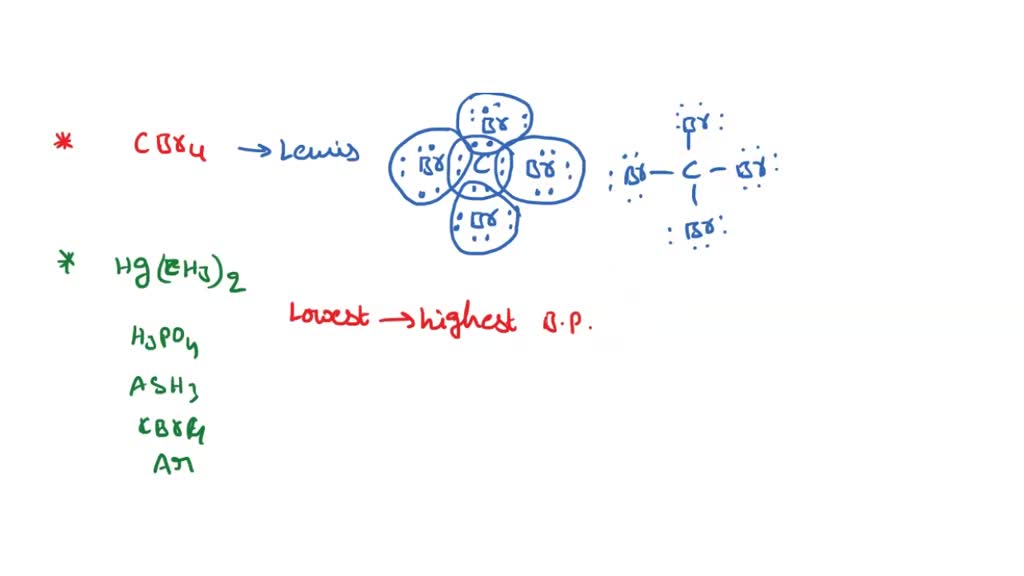
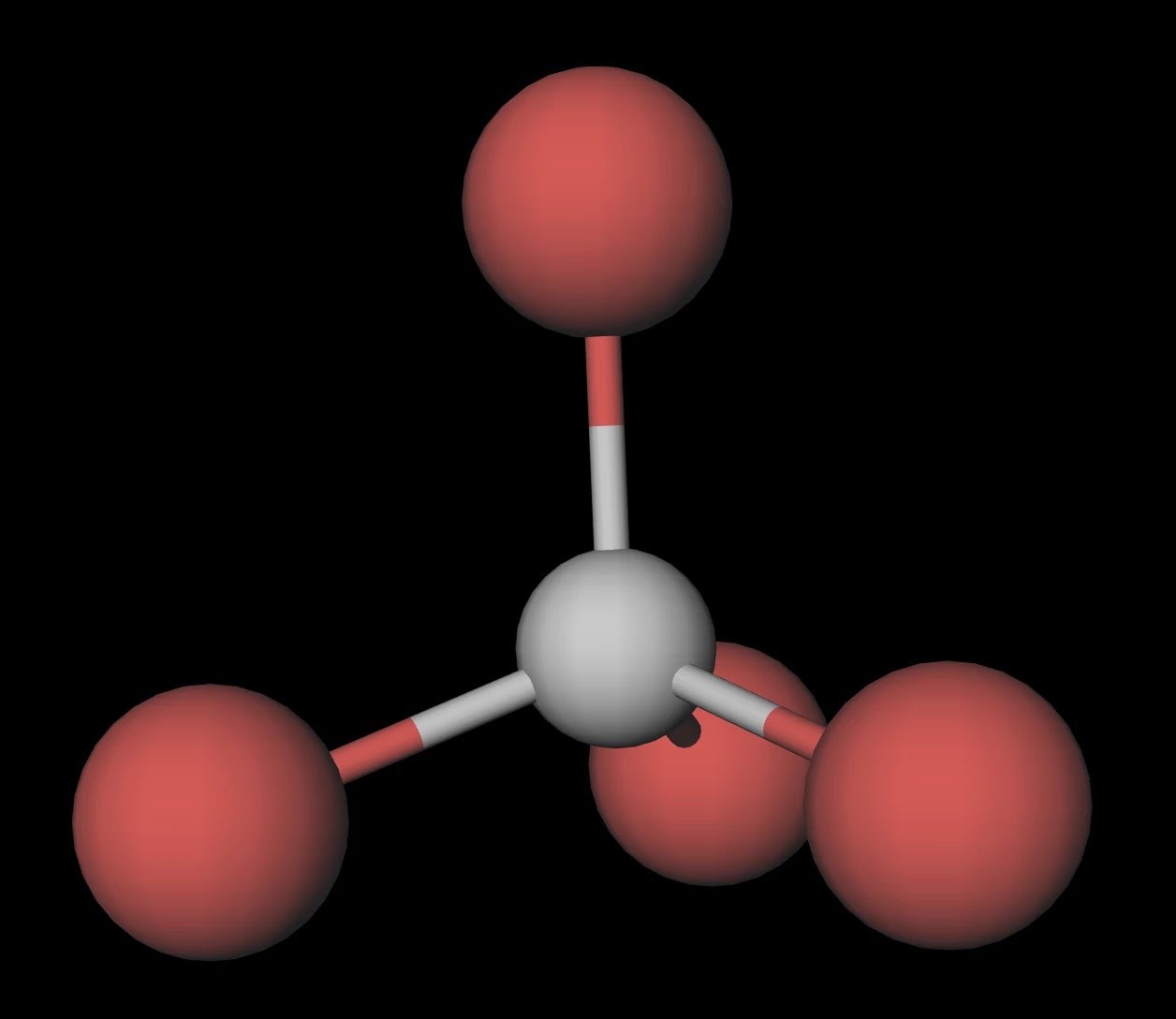
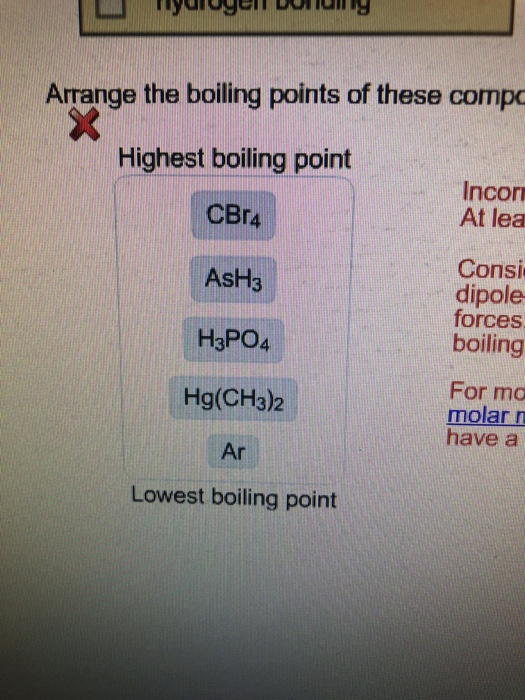
SOLVED: Draw the Lewis structure for carbon tetrabromide, CBr4. Include lone pairs. Arrange the compounds from lowest boiling point to highest boiling point. • Hg(CH3)2 • H3PO4 • AsH3 •CBr4 •Ar

SOLVED: Formula CBr4 CH2Br2 Boiling Point (C) 94.5 98.0 Vapor Pressure at 20c (kPa) 5.33 4.65 romide ane ibromomethane has a greater solubility in water than carbon tetrabromide has ccount for this