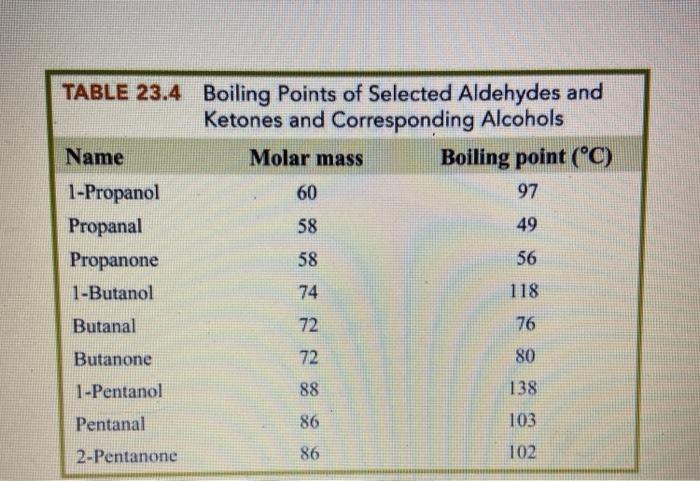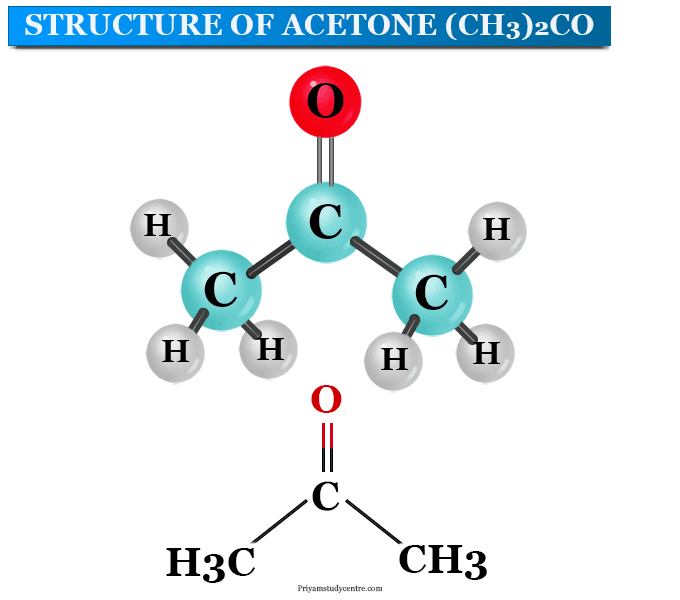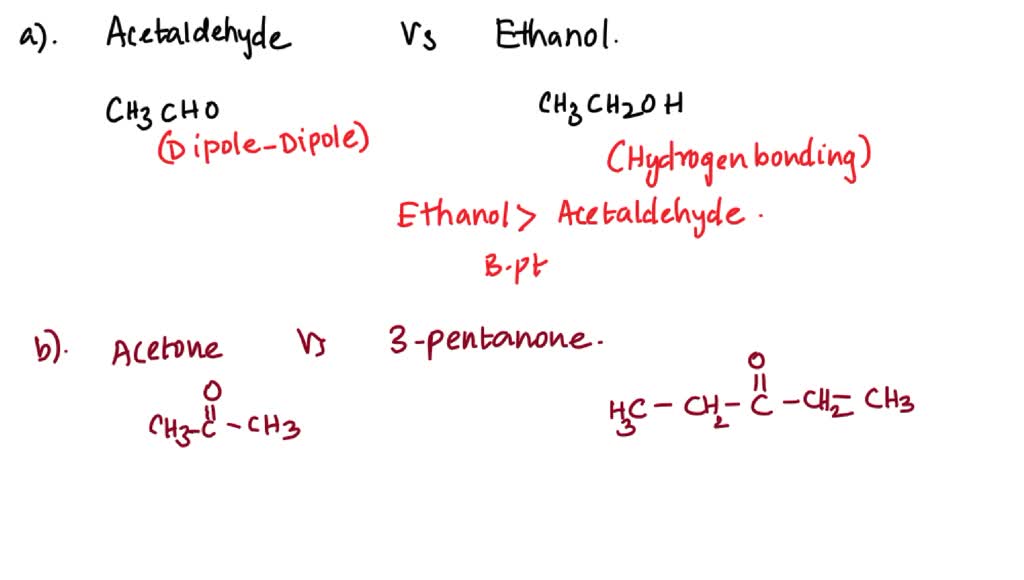
SOLVED: In each pair of compounds, select the one with the higher boiling point. (a) Acetaldehyde or ethanol (b) Acetone or 3 -pentanone (c) Butanal or butane (d) Butanone or 2-butanol
Why does acetone has a higher boiling point than propanal if they both have the same molecular mass? - Quora

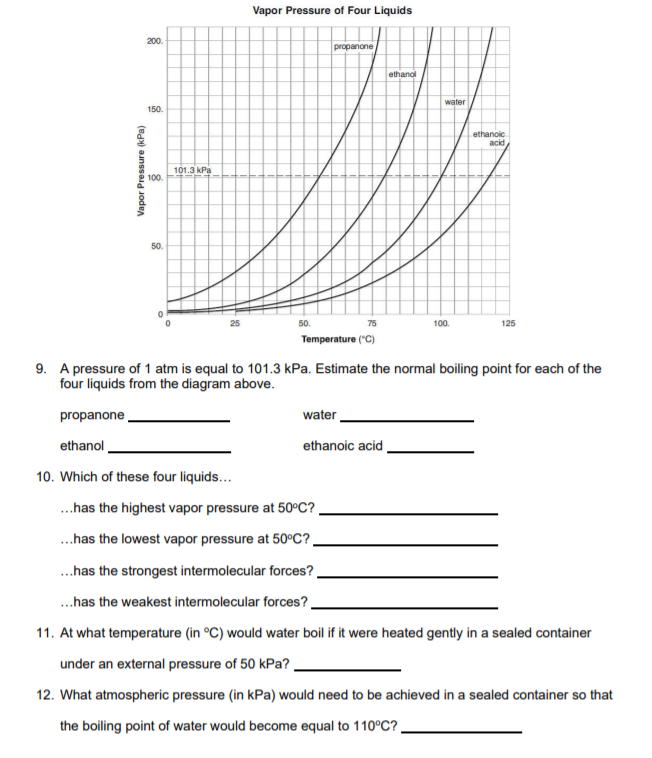
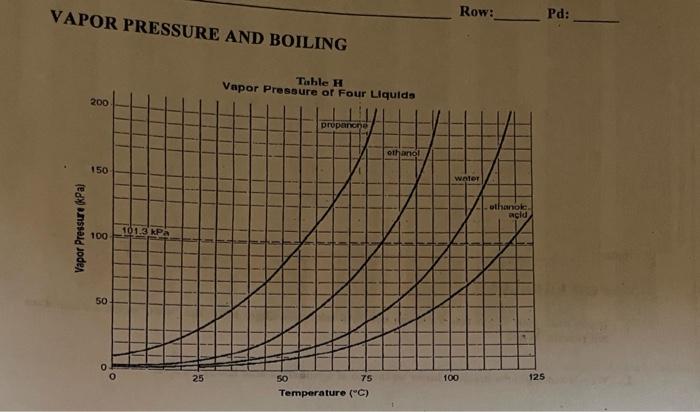
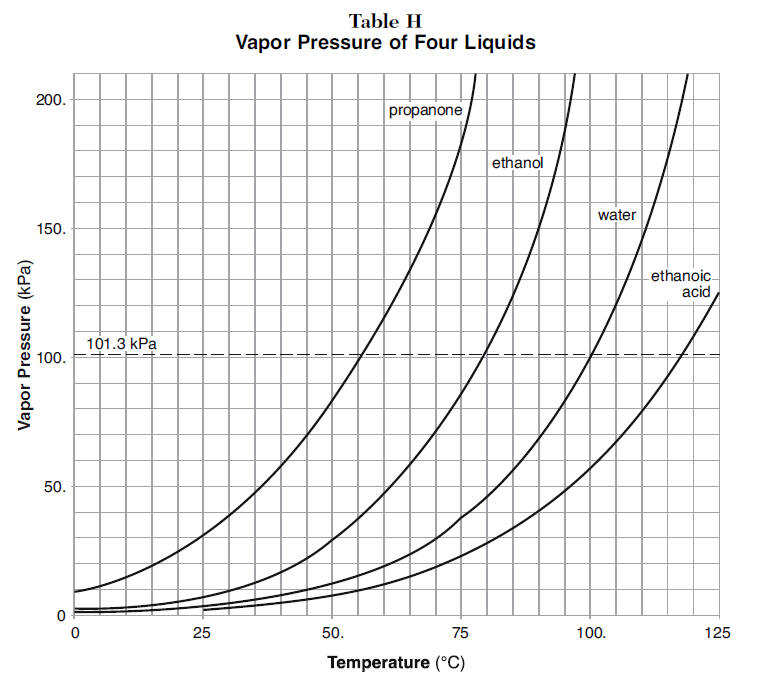
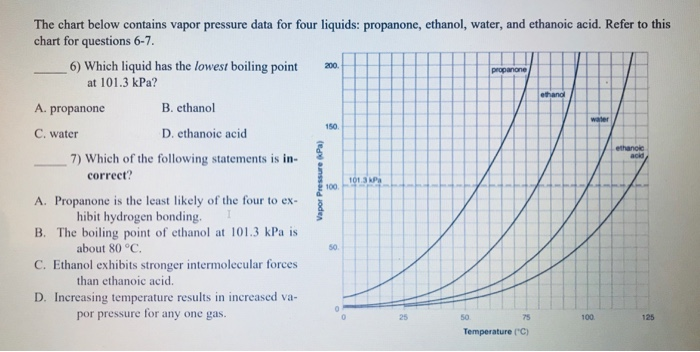
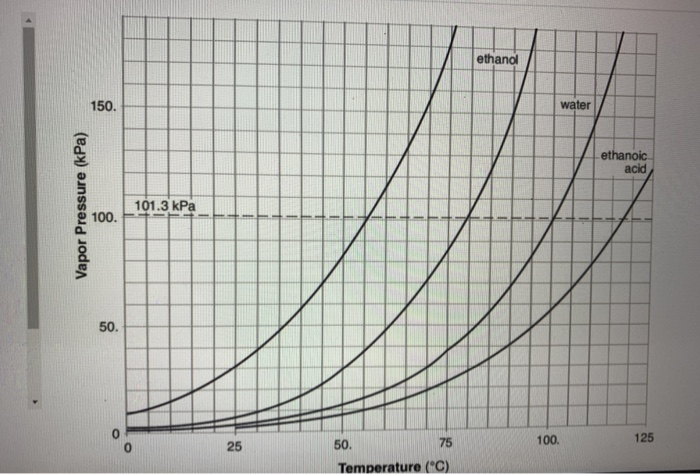
Propanone, Boils at 68 o C, at 150 kPa Ethanol, Boils at 90 o C, at 150 kPa Water, Boils at 110 o C, at 150 kPa LOGIC: Propanone exerts 150 kPa at ppt download
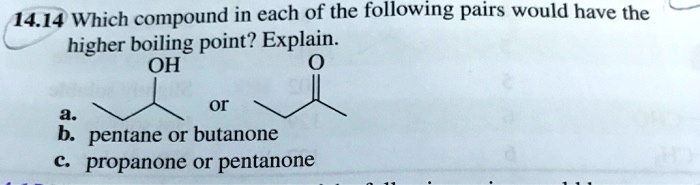
SOLVED: 14.14 Which compound in each of the following pairs would have the higher boiling point? Explain. OH or b: pentane or butanone propanone or pentanone

Arrange the following in increasing order of boiling points: - Chemistry - Aldehydes Ketones and Carboxylic Acids - 15104087 | Meritnation.com
How to calculate the boiling point of a mixture made up of 2 liquids ( acetone+water in the same ratios) - Quora

The boiling point of pure acetone is 56.38^(@)C`. When 0.707 g of a compound is dissolved in 10 g of - YouTube