Catalytic Dehydration of Carbohydrates Suspended in Organic Solvents Promoted by AlCl3/SiO2 Coated with Choline Chloride - Yang - 2015 - ChemSusChem - Wiley Online Library

The correct relationship between the boiling points of very dilute solutions of AlCl3(t1) and CaCl2(t2) , having the same molar concentration, is :

Experimental investigation on the direct crystallization of high-purity AlCl3·6H2O from the AlCl3-NaCl-H2O(-HCl-C2H5OH) system - ScienceDirect
Survey of Properties of Key Single and Mixture Halide Salts for Potential Application as High Temperature Heat Transfer Fluids f

Determine the boiling points of 1 m solution of sugar, glucose, urea, sodium chloride, barium chloride, aluminium chloride. | Homework.Study.com
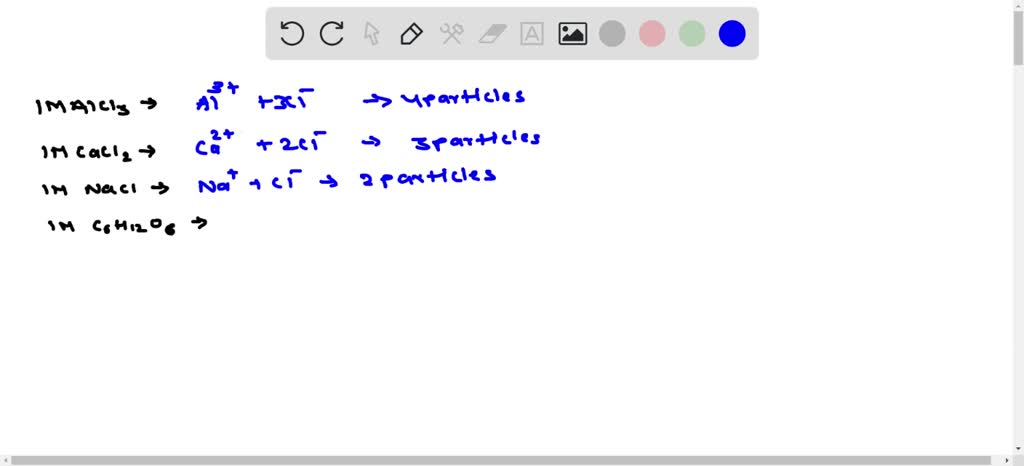
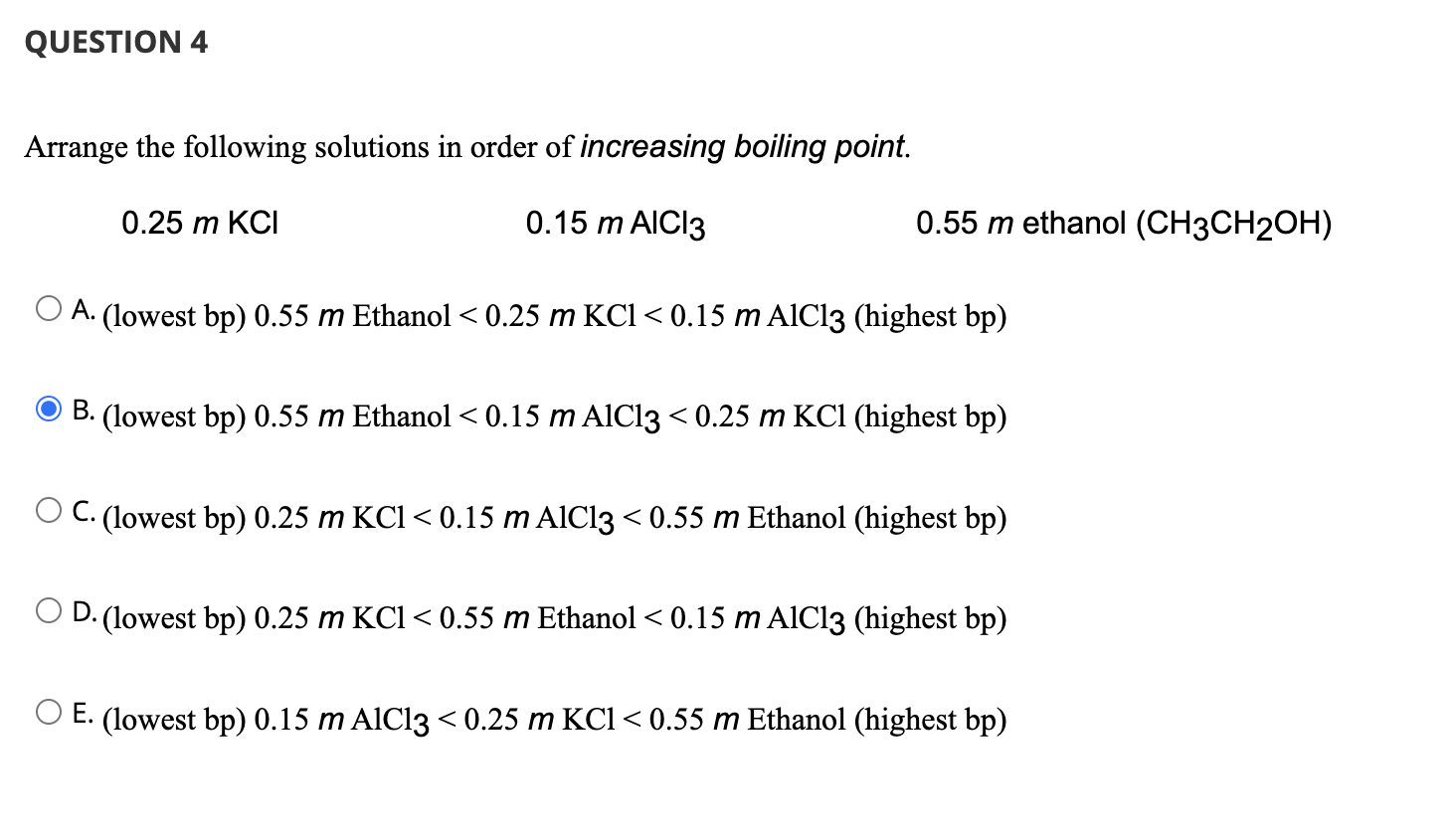
SOLVED: Arrange the following according to increasing boiling point and freezing point. 1m AlCl3, 1m CaCl2, 1m NaCl, 1m C6H12O6 Increasing Boiling Point Increasing Freezing Point

The correct relationship between the boiling points of very dilute solutions of AlCl3(T1) and CaCl2(T2) , having the same molar concentration is:

Pressure-temperature phase diagram of AlCl 3 including (extrapolation... | Download Scientific Diagram
Molality Chart Problem Problem Convert Convert Colligative Properties Where it is used Boiling Water

Suppose you had a 1.00 m solution of AlCl_3. Assuming complete dissociation, what is the theoretical change in the freezing point of this solution? | Homework.Study.com
![SOLVED: Sometimes Aluminum Chloride [AlCl3] is used to address excessive sweating. Given the following information please answer the following question Kf=1.80 C/m Kb= 0.512 C/m What is the new boiling point when SOLVED: Sometimes Aluminum Chloride [AlCl3] is used to address excessive sweating. Given the following information please answer the following question Kf=1.80 C/m Kb= 0.512 C/m What is the new boiling point when](https://cdn.numerade.com/project-universal/previews/55f23ef4-8e27-4a18-89f1-c8e26ba39fb8.jpg)